In times of strict practices of social distancing, non-essential work cessation and quarantining, conducting life-saving clinical research can be a challenge.
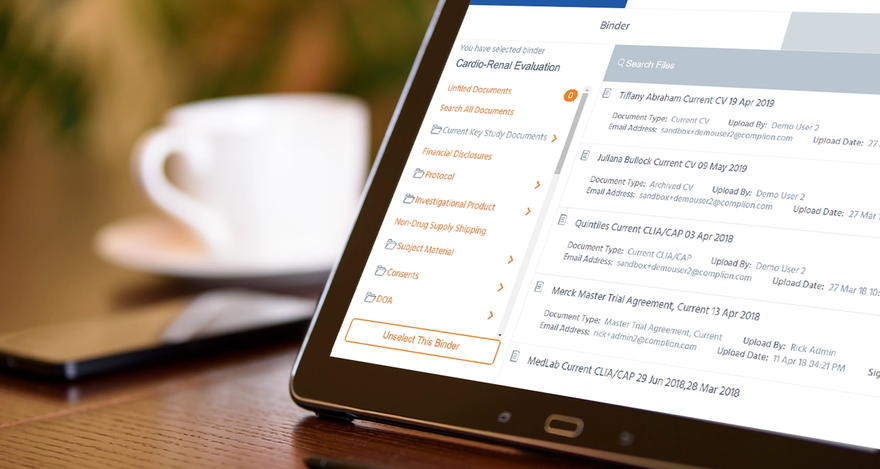
For some research sites, operations may grind to a halt without key team members in the office to conduct regulatory business. For others, it is nearly impossible for sponsors and monitors to conduct necessary regulatory oversight. But there is a solution, offered by Cleveland-based Complion, which helps with the regulatory filing needs of clinical research sites, during the pandemic and after. Its software platform that allows research teams to securely and virtually:
- Enable monitors. Monitors can remotely review digital regulatory documentation without interrupting the site coordinators.
- Work remotely/distanced/digitally. A number of sites, to follow regional orders or best practices, are limiting onsite staff to essential workers and encouraging staff to work remotely or to work with patients remotely. Important documents can still be managed, updated, shared and signed – CVs need updating, or protocols trainings need to be completed – easily, digitally and wherever clinical or regulatory staff are located, including the collection of compliant signatures.
- Prepare for the future. The onslaught of work when pandemic conditions lift may be overwhelming for some teams, which is why implementing tools for increasingly effective and efficient regulatory document management is important now.

At a time when every research team must change the way it works, Complion’s platform provides digital and remote storage, retrieval and sharing of regulatory documentation as well as compliant e-signatures to help staff easily manage work and keep life-saving research trials on time and budget. To help clinical research sites start using the Complion suite of tools quickly, they are offering a fast-track implementation process and unlimited users in the system for no additional charge. To begin safe, remote implementation of the Complion system with your clinical research team, please call 800-615-9077 or email daysia.hadden@complion.com.